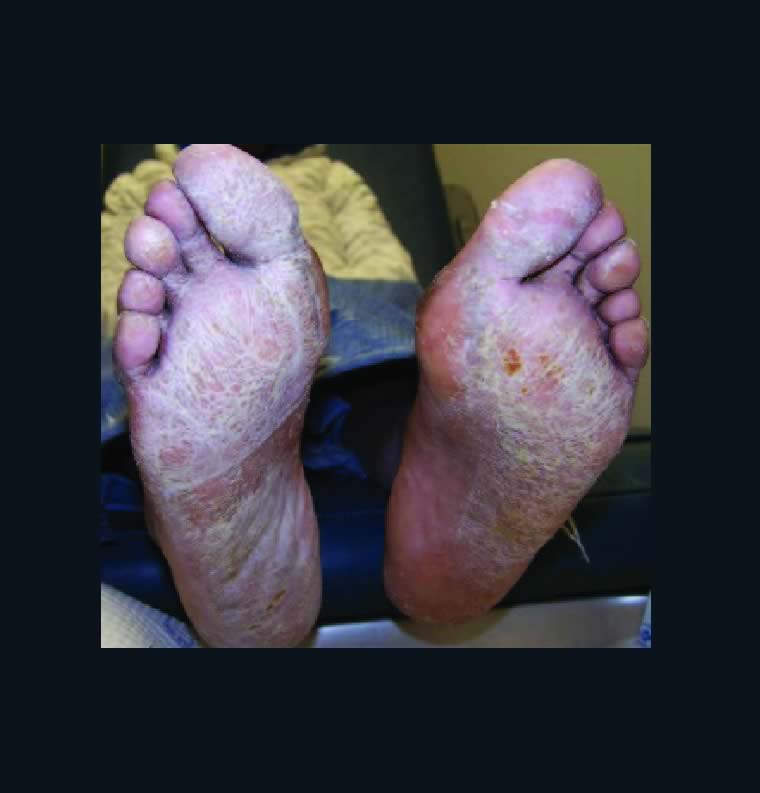
Given the common nature of xerotic skin disorders as well as the varied array of etiologies and treatments, these authors offer a thorough review of the literature on conditions ranging from ichthyosis and atopic dermatitis to venous stasis dermatitis and asteatotic dermatitis.
Xerosis is a very common skin disorder characterized by excessively dry skin. Other terms for this disorder include xerosis cutis and xeroderma. Xerosis can be a primary pathology associated with loss of the normal water content of the epidermis. Xerotic skin can also occur secondary to associated skin disorders and systemic disease. Underlying all xerotic skin disorders is excess water loss from the epidermis.
Skin requires a water content of 10 to 15 percent to remain intact and maintain normal function.1 Three main deficiencies in the skin lead to the development of xerosis including deficiency in natural moisturizing factors; deficiency in the skin lipids or ceramides; and deficiency in moisture in the epidermis that is mediated by aquaporin water channels.2-7 Natural moisturizing factors are isolated to the stratum corneum in high concentration in the corneocytes. These factors consist of amino acids and their derivatives including lactate, urea and inorganic salts.2 Lipids in the stratum corneum modulate water loss. Deficiencies of these cutaneous lipids can increase epidermal water loss up to 75 times that of normal skin.8
Ceramides are the main lipids in the stratum corneum. Numerous risk factors contribute to loss of cutaneous lipids and predispose individuals to develop xerotic skin disorders. This may include decreased sebaceous and sweat gland activity associated with aging; anti-androgen therapy, which decreases sebum production; exposure to degreasing agents including soaps and solvents; and exposure to dry environments.
Xerosis has variable presentation depending on its severity. Mild xerosis can exhibit accentuation of skin lines and resemble the appearance of cracked porcelain due to epidermal water loss. Xerosis affects the normal desquamation process of the epidermis, leading to the development of thin flakes on the skin surface. With more severe xerosis, one will see pruritic, dry, cracked and fissured skin. Severe xerosis can produce an inflammatory dermatitis with localized erythema and edema. Clinicians may note xerotic skin on numerous areas of the body including the lower extremity, upper extremity, abdomen and face.
Patients of increased age are at significantly higher risk of developing xerotic skin disorders.9 Sebaceous gland activity decreases significantly after 70 years of age in women and 80 years of age in men.10 Sweat gland function also declines with age.11 Skin thickness decreases with age, leading to increased water loss from the skin to the environment.12 Environmental factors are also significant risk factors for the development of xerosis. In winter months when humidity decreases, xerosis occurs much more frequently. Xerotic skin disorders are more common in dry climates with low humidity.
Basic treatment for all xerotic skin disorders aims to minimize cutaneous water loss. Lazar and Lazar identified the following methods to prevent water loss and lubricate the skin:
• reduce the frequency of bathing, showering and skin cleansing;
• increase room humidity;
• limit exposure to soaps, detergents, solvents and water;
• avoid friction from washcloths, clothing and other abrasives; and
• use emollients frequently.13
Moisturizers are a mainstay in the treatment of xerotic skin. The skin contains natural moisturizers including ceramides, glycerol, urea and lactic acid. Many moisturizers contain these elements aiming to supplement these natural moisturizing agents. Skin care products that both improve skin hydration and improve barrier function are wise choices. Specific products should contain both rehydrating and lipid-restoring components. Urea has the largest body of evidence for the treatment of xerosis.14 Combining urea with moisturizing agents and ceramides can improve its effectiveness.
Aiming to address multiple key deficiencies in skin hydration, Weber and colleagues formulated a topical formulation containing glyceryl glucoside, natural moisturizing factors and ceramide, and found it to be an effective treatment modality for xerosis.15
Addressing Asteatotic Dermatitis And Ichthyosis In The Podiatric Patient
Asteatotic dermatitis is an inflammatory dermatitis secondary to severely xerotic skin. Other terms for this disorder include xerotic dermatitis, xerotic eczema and eczema craquelé. Asteatotic dermatitis most commonly occurs in elderly people with underlying xerosis.
Asteatotic dermatitis can be generalized or localized. Generalized disease is often associated with underlying systemic disease. Localized forms most commonly occur on the pretibial areas. Patients with asteatotic dermatitis exhibit dry, cracked and polygonal fissured skin with scaling and pruritis. Secondary erythema, edema and excoriations can develop from scratching. Fissures with superficial bleeding can occur when the skin develops cracks deep enough to damage dermal capillaries.
Known as “winter itch,” asteatotic dermatitis most commonly occurs in the winter months when environmental humidity is the lowest. Asteatotic dermatitis is prevalent in the elderly due to decreased sebaceous and sweat gland activity associated with aging. Aside from climate and age, certain medications, including diuretics, retinoids and protein kinase inhibitors, can also contribute to the development of asteatotic dermatitis.16
In addition to the preventative skin care recommended by Lazar and Lazar, topical steroid ointments under occlusion and Unna boots are treatment options for asteatotic eczema.13,17 Topical calcineurin inhibitors, including pimecrolimus and tacrolimus cream, show efficacy in the treatment of asteatotic dermatitis.18 Recently, endogenous phospholipids, N-palmitoylethanolamine and N-acetylethanolamine, that are part of the endocannabinoid system have proven to be effective treatments for asteatotic dermatitis with efficacy superior to traditional emollients.19
Ichthyosis is a group of skin disorders characterized by excessive dry, scaling skin. The name for this disorder comes from the Greek word, ichthys, meaning fish, since this disorder is known for its xerotic scales. Both inherited and acquired forms of ichthyosis exist with the most common form being ichthyosis vulgaris, an inherited autosomal-dominant disorder that commonly begins in childhood.20 Patients with ichthyosis vulgaris have xerotic skin with fine white scales. Scaling is most common on the extensor surfaces of the extremities. Acquired ichthyosis typically occurs in adults and is associated with medications that inhibit sterol synthesis in epidermal cells (nicotinic acid) or underlying systemic diseases including Hodgkin’s lymphoma, leukemia, sarcoidosis, human immunodeficiency virus (HIV), hypothyroidism, hepatitis, malabsorption and bone marrow transplantation.21 Acquired ichthyosis appears as small white scales on the extremities.
Clinicians may treat ichthyosis with topical creams and emollients to hydrate the skin and keratolytics to remove scales.
Creams containing a high percentage of urea or lactic acid can be very effective treatment options for ichthyosis.22 Oral retinoids such as acitretin (Soriatane) and isotretinoin have a general anti-keratinizing effect, and the literature suggests effectiveness in the treatment of more severe cases of ichthyosis.20
What Are The Best Approaches For Atopic And Venous Stasis Dermatitis?
Atopic dermatitis is an inflammatory skin disorder, which is often associated with xerotic skin. This disorder presents as dry, itchy, red, swollen and cracked skin. There is often serous drainage and the presentation can vary with age. A total body distribution is more typical in infancy. For children, it is more common to see atopic dermatitis in the back of the knees and the front of the elbows. The feet and hands are the most common sites in adults.
Frequently, atopic dermatitis is associated with allergies and asthma. Several factors are thought to contribute to the development of atopic dermatitis including genetics, immune system dysfunction, environmental triggers and disruption of skin permeability. Dry skin secondary to dry climate, frequent washing and harsh chemicals increases the risk of developing atopic dermatitis.23
Treatment of atopic dermatitis varies based on the severity of the disease. Basic treatment involves avoiding aggravating environments and keeping the skin moist with moisturizers and emollients.24 Mild to moderate disease may respond to topical corticosteroids.25 Oral corticosteroids and calcineurin inhibitors are applicable for the treatment of more severe and resistant cases.23,26-28
Venous stasis dermatitis is a common inflammatory disorder affecting the skin of the lower extremities. It is frequently one of the first manifestations of chronic venous insufficiency, when retrograde blood flow through incompetent valves leads to venous hypertension and the eventual extravasation of red blood cells and ferric iron into dermal tissues. Dermal tissue changes results both directly from venous hypertension and from an inflammatory process mediated by metalloproteinases that are upregulated by ferric iron in extravasated red blood cells.29
Stasis dermatitis appears as erythematous, scaling, eczematous patches on the lower extremity. The medial ankle is the most common site, owing to its relatively poor blood supply. Skin lesions can vary in distribution from small patches to areas encompassing the entire lower leg below the knee and involving the dorsal foot. Long-standing skin lesions can present with lichenification and hyperpigmentation. Additionally, chronic venous insufficiency and hypertension can lead to skin induration and progression to lipodermatosclerosis.30
The treatment of stasis dermatitis involves management of the underlying venous insufficiency and edema. One typically treats this condition through compression therapy.29 Xerotic skin in areas of quiescent dermatitis often responds to emollients and moisturizers. Mid-potency topical steroids are applicable for short durations in the management of acute inflammation and pruritus. Long-term and high-potency topical corticosteroids are not desirable as they can lead to steroid-induced cutaneous atrophy, which can increase the risk of developing venous skin ulcerations.31,32
While topical calcineurin inhibitors are only approved for the treatment of atopic dermatitis, they are reportedly effective treatment modalities for many inflammatory skin disorders including stasis dermatitis.33,34 Tacrolimus has specifically proven effective in the treatment of stasis dermatitis.35 Maroo and colleagues found a combination of topical tacrolimus and oral doxycycline to be effective for stasis dermatitis.36
What Is The Relationship Between Systemic Disease And Xerotic Skin?
Several systemic diseases can cause xerosis and the workup of xerotic skin changes should include consideration of underlying systemic disease. Disorders including diabetes mellitus, thyroid disease and severe renal disease are frequently associated with xerotic skin. Treatment of xerosis secondary to systemic disease typically involves management of the underlying disease state as well as symptomatic management.
It is common to observe xerotic skin in patients with diabetes mellitus. Dry skin has the potential to fissure, increasing the risk of foot ulceration and infection in patients with diabetes mellitus.37,38
The nervous system plays an important role in maintaining adequate skin hydration. Diabetic polyneuropathy affects small sympathetic nerves, resulting in atrophy of sweat glands and decreased sudomotor response.39-42
Additionally, microcirculatory disease in patients with diabetes can lead to dry, rough, atrophic skin. Namgoong and team specifically examined the effect of peripheral neuropathy and microangiopathy on skin hydration in the feet of patients with diabetes mellitus.43 These researchers found a significant correlation between skin hydration and microvascularity, but no significant correlation between skin hydration and peripheral nerve function.
Hypothyroidism is a disorder of the endocrine system in which the thyroid gland fails to produce adequate amounts of thyroid hormone. Thyroid dysfunction is more common in women and people over the age of 60. This underproduction of thyroid hormones decreases the activity of the sweat glands, resulting in dry, xerotic skin.44 Skin changes in hypothyroidism include coarse, thin, scaly skin.45 The prevailing theory is that reduction of thyroid hormone alters sterol synthesis in epidermal keratinocytes, leading to xerotic skin changes.46 Treatment of hypothyroid-associated skin changes involves treatment of the underlying endocrine disorder with thyroid hormone supplementation.
Skin disorders are also extremely common in patients with chronic renal failure (CRF) and end-stage renal disease (ESRD).47 Xerosis is the most common skin disorder associated with renal disease, reportedly occurring in over 80 percent of patients with chronic renal failure.48 When it comes to the development of xerosis in chronic renal failure and ESRD, researchers have proposed several etiologies including decreased sweat production, decreased sebum production, reduced lipids in the skin surface, altered vitamin A metabolism, loss of or reduction in epidermal water content, and disruption of the integrity of the stratum corneum.49,50
In chronic renal failure and ESRD, reduced glomerular filtration rate leads to accumulation of waste products, including urea, creatinine, sodium, calcium, and phosphate, that are some of the main agents associated with the pathogenesis of skin disease in severe renal disease.51 Patients with severe xerosis secondary to renal disease can develop ichthyosis. Moisturizers with 5-10% urea cream or 2-3% salicylic acid are options for the treatment of uremic xerosis.49,52
In Conclusion
Xerotic skin disorders are very common and have numerous etiologies including local and systemic disease. Both age and environmental factors play significant roles in the development of these disorders. Management of xerotic skin varies based on severity and pathology, and frequently involves management of environmental risk factors, emollients and moisturizers, and treatment of underlying disease states.
Dr. Hoffman is an Attending Physician in the Department of Orthopedics at Denver Health Medical Center. She is an Assistant Professor in the Department of Orthopedics at the University of Colorado School of Medicine. She is an Attending Physician for the Highland/Presbyterian St. Luke’s Medical Center Residency Program.
Dr. Jerabek is an Attending Physician in the Department of Orthopedics at Denver Health Medical Center. She is an Assistant Professor in the Department of Orthopedics at the University of Colorado School of Medicine.
1. Pons-Guiraud A. Dry skin in dermatology: a complex physiopathology. J Eur Acad Dermatol Venereol. 2007;21 Suppl 2:1-4.
2. Rawlings AV, Scott IR, Harding CR, Bowser PA. Stratum corneum moisturization at the molecular level. J Invest Dermatol. 1994;103(5):731- 741.
3. Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17 Suppl 1:43-48.
4. Jungersted JM, Hellgren LI, Jemec GB, Agner T. Lipids and skin barrier function–a clinical perspective. Contact Dermatitis. 2008;58(5):255- 262.
5. Elias PM, Feingold KR. Lipids and the epidermal water barrier: metabolism, regulation, and pathophysiology. Semin Dermatol. 1992;11(2):176-182.
6. Draelos ZD. New channels for old cosmeceuticals: aquaporin modulation. J Cosmet Dermatol. 2008;7(2):83.
7. Bonte F. Skin moisturization mechanisms: new data. Ann Pharm Fr. 2011;69(3):135-141.
8. Akimoto K, Yoshikawa N, Higaki Y, Kawashima M, Imokawa G. Quantitative analysis of stratum corneum lipids in xerosis and asteatotic eczema. J Dermatol. 1993;20(1):1-6.
9. White-Chu EF, Reddy M. Dry skin in the elderly: complexities of a common problem. Clin Dermatol. 2011;29(1):37-42.
10. Pochi PE, Strauss JS, Downing DT. Age-related changes in sebaceous gland activity. J Invest Dermatol. 1979;73(1):108-111.
11. Anderson RK, Kenney WL. Effect of age on heat-activated sweat gland density and flow during exercise in dry heat. J Appl Physiol. 1987;63(3):1089-1094.
12. Farage MA, Miller KW, Elsner P, Maibach HI. Characteristics of the aging skin. Adv Wound Care (New Rochelle). 2013;2(1):5-10.
13. Lazar AP, Lazar P. Dry skin, water, and lubrication. Dermatol Clin. 1991;9(1):45-51.
14. Augustin M, Wilsmann-Theis D, Korber A, et al. Diagnosis and treatment of xerosis cutis – a position paper. J Dtsch Dermatol Ges. 2019;17 Suppl 7:3-33.
15. Weber TM, Kausch M, Rippke F, Schoelermann AM, Filbry AW. Treatment of xerosis with a topical formulation containing glyceryl glucoside, natural moisturizing factors, and ceramide. J Clinical Aesth Dermatol. 2012;5(8):29- 39.
16. Norman R. Xerosis and pruritus in the elderly—recognition and management. In: Norman R, ed. Diagnosis of Aging Skin Diseases. London: Springer London; 2008.
17. Ward S. Eczema and dry skin in older people: identification and management. Br J Community Nurs. 2005;10(10):453-456.
18. Schulz P, Bunselmeyer B, Brautigam M, Luger TA. Pimecrolimus cream 1% is effective in asteatotic eczema: results of a randomized, double-blind, vehicle-controlled study in 40 patients. J Eur Acad Dermatol Venereol. 2007;21(1):90-94.
19. Yuan C, Wang XM, Guichard A, et al. N-palmitoylethanolamine and N-acetylethanolamine are effective in asteatotic eczema: results of a randomized, double-blind, controlled study in 60 patients. Clin Interv Aging. 2014;9:1163- 1169.
20. Vahlquist A, Fischer J, Torma H. Inherited nonsyndromic ichthyoses: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2018;19(1):51-66.
21. DiGiovanna JJ, Robinson-Bostom L. Ichthyosis: etiology, diagnosis, and management. Am J Clin Dermatol. 2003;4(2):81-95.
22. Blair C. The action of a urea-lactic acid ointment in ichthyosis with particular reference to the thickness of the horny layer. Br J Dermatol. 1976;94(2):145-153.
23. Tollefson MM, Bruckner AL, Section On Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134(6):e1735-1744.
24. Varothai S, Nitayavardhana S, Kulthanan K. Moisturizers for patients with atopic dermatitis. Asian Pac J Allergy Immunol. 2013;31(2):91-98.
25. Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Phys. 2012;86(1):35- 42.
26. Ashcroft DM, Chen LC, Garside R, Stein K, Williams HC. Topical pimecrolimus for eczema. Cochrane Database Syst Rev. 2007(4):CD005500.
27. Cury Martins J, Martins C, Aoki V, Gois AF, Ishii HA, da Silva EM. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015(7):CD009864.
28. Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15(4):303-310.
29. Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18(3):383-390.
30. Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol. 1993;28(4):623-627.
31. Wilkinson SM, English JS. Hydrocortisone sensitivity: clinical features of fifty-nine cases. J Am Acad Dermatol. 1992;27(5 Pt 1):683-687.
32. Lubach D, Bensmann A, Bornemann U. Steroid-induced dermal atrophy. Investigations on discontinuous application. Dermatologica. 1989;179(2):67-72.
33. Lin AN. Innovative use of topical calcineurin inhibitors. Dermatol Clin. 2010;28(3):535-545.
34. Wollina U. The role of topical calcineurin inhibitors for skin diseases other than atopic dermatitis. Am J Clin Dermatol. 2007;8(3):157-173.
35. Dissemond J, Knab J, Lehnen M, Franckson T, Goos M. Successful treatment of stasis dermatitis with topical tacrolimus. Vasa. 2004;33(4):260-262.
36. Maroo N, Choudhury S, Sen S, Chatterjee S. Oral doxycycline with topical tacrolimus for treatment of stasis dermatitis due to chronic venous insufficiency: A pilot study. Indian J Pharmacol. 2012;44(1):111-113.
37. Papanas N, Maltezos E. The diabetic foot: established and emerging treatments. Acta Clin Belg. 2007;62(4):230-238.
38. Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24 Suppl 1:S3-6.
39. Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34(1):57-61.
40. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285-2293.
41. Papanas N, Ziegler D. New diagnostic tests for diabetic distal symmetric polyneuropathy. J Diabetes Complications. 2011;25(1):44-51.
42. Papanas N, Boulton AJ, Malik RA, et al. A simple new non-invasive sweat indicator test for the diagnosis of diabetic neuropathy. Diab Med. 2013;30(5):525-534.
43. Namgoong S, Yang JP, Han SK, Lee YN, Dhong ES. Influence of peripheral neuropathy and microangiopathy on skin hydration in the feet of patients with diabetes mellitus. Wounds. 2019;31(7):173-178.
44. Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3(3):211-215.
45. Kohn LT, Corrigan JM, Donaldson MS (eds). To Err is Human: Building a Safer Health System. Washington, DC: Institute of Medicine: National Academies Press; 2000. DOI: 10.17226/9728
46. Rosenberg RM, Isseroff RR, Ziboh VA, Huntley AC. Abnormal lipogenesis in thyroid hormone-deficient epidermis. J Invest Dermatol. 1986;86(3):244-248.
47. Amatya B, Agrawal S, Dhali T, Sharma S, Pandey SS. Pattern of skin and nail changes in chronic renal failure in Nepal: a hospital-based study. J Dermatol. 2008;35(3):140-145.
48. Sheikh M ML, Jahangir M. Cutaneous manifestations of chronic renal failure. J Pakistan Assn Dermatol. 2014;24(2):150-155.
49. Szepietowski JC, Reich A, Schwartz RA. Uraemic xerosis. Nephrol Dial Transplant. 2004;19(11):2709-2712.
50. Lupi O, Rezende L, Zangrando M, et al. Cutaneous manifestations in end-stage renal disease. An Bras Dermatol. 2011;86(2):319-326.
51. Galperin TA, Cronin AJ, Leslie KS. Cutaneous manifestations of ESRD. Clin J Am Soc Nephrol. 2014;9(1):201-218.
52. Kuypers DR. Skin problems in chronic kidney disease. Nat Clin Pract Nephrol. 2009;5(3):157- 170.